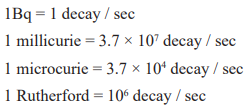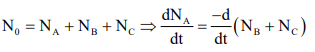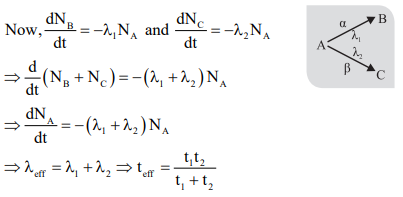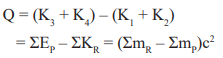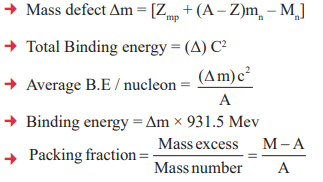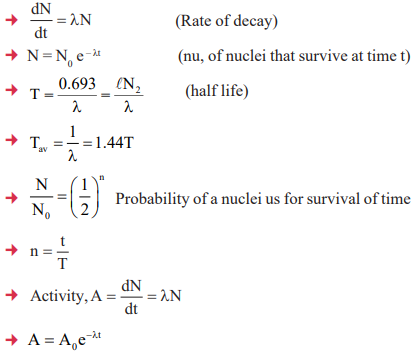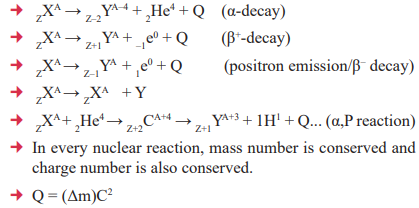Hi CUET aspirants, Welcome to Amans Maths Blogs (AMBIPi). In this post, you will get CUET Physics Study Materials Atoms and Nuclei Notes AMBIPi. This CUET Physics Notes are designed by analyzing to the CUET Syllabus and CUET Previous Years Questions Papers.
CUET Physics Notes
CUET Physics Dual Nature of Radiation and Matter: Important Points to Remember
There are following important points in this chapter of Atoms and Nuclei.
- Bohr’s model is applicable only to hydrogenic atoms (single electron).
- Thomson’s model of atom could not explain the origin of spectral series of hydrogen and other atoms, observed experimentally.
- An alpha particle is helium nucleus containing 2 protons and 2 neutrons. Therefore, an alpha particle has 4 units of mass and 2 units of positive charge.
- In Rutherford’s experiment, only about 0.14% of incident α-particles scatter by more than 1o.
- About 1α-particle in every 8000α-particles deviate by more than 90o.
- In Rutherford’s α-ray scattering experiment, the scattering is due to elastic collision between nucleus and α-particle.
- Distance of closest approach is that distance from nucleus at which K.E of α-particle reduces to zero and particle stop and cannot go closer to the nucleus and retraces its path by 180o.
- If the electron in an atom were stationary they would fall into the nucleus on account of electrostatic force of attraction between electrons and the nucleus.
- Angular momentum dimensions are the same as those of Planck’s constant.
- X-rays are produced by bombarding high speed electrons on a target of high atomic weight and high melting point.
- X-rays are Short wavelength (0.1Å to 10Å) electromagnetic radiation.
- X-rays are produced when a metal anode is bombarded by very high energy electrons.
- X-rays are not affected by electric and magnetic field.
- X-rays cause photoelectric emission.
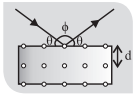
- Diffraction of X-ray take place according to Bragg’s law 2d sinθ = nλ.
where, d = spacing of crystal plane or lattice constant or distance between adjacent atomic plane, θ = Bragg’s angle or glancing angle, ϕ = Diffracting angle n = 1, 2, 3 …..
- For maximum Wavelength: sin θ = 1, n = 1 ⇒ λmax = 2d so if λ = 2d diffraction is not possible i.e., solution of Bragg’s equation is not possible.
- Neutrons were discovered by Chadwick, which is present in nucleus of the atom.
- Volume of nucleus is about 10–12 times the volume of the atom. In other words atom is almost empty.
- Nucleon word / term refers for the proton or a neutron.
- A free neutron, unlike a free proton, is unstable. It decays into a proton, an electron and a antineutrino (another
elementary particle), and has a mean life of about 1000s. - The density of nuclear matter is approximately 2.3 × 1017 Kgm-3.
- The difference in mass of a nucleus and its constituents ΔM, is called the mass defect.
- For the average mass nuclei, the binding energy per nucleon is approximately 8 Mev.
- The difference between a proton and a neutron is essentially in the composition of their respective meson clouds.
- S.I unit of radioacitvity is Becquerel (Bq)
- In nuclear reactions the following conservation laws are obeyed.
- In α-decay, an α-particle is emitted from parent nuclei and their mass number decreases by 4 and atomic number
decrease by 2. - In β minus decay, transformation of neutron into a proton, mass number decreases by one, z increases by one but
atomic mass does not change. - In, β+ decay (β plus), orbital electron can combine with a proton in the nucleus to form a neutron and neutrino, mass number increases by one and atomic number (z) decreases by one but atomic mass remains same.
- If the relative abundance of isotopes in an element has a ratio n1 : n2 whose atomic masses are m1 and m2 then atomic mass of element is
.
- Parallel Radioactive Disintegration: Let initial number of nuclei of A is No then at any time number of nuclei of A, B & C are given by
A disintegrates into B and C by emitting α, β particle.
- Nuclear Collisions: We can represent a nuclear collision or reaction by the following notation, which means X(a,b) Y
- Energy released in any nuclear reaction or collision is called Q value of the reaction.
-
If Q is positive, energy is released and products are more stable in comparison to reactants.
- If Q is negative, energy is absorbed and products are less stable in comparison to reactants.
- Nuclear Fission: In 1938 by Hahn and Strassmann, By attach of a particle splitting of a heavy nucleus (A > 230) into two or more lighter nuclei. In this process certain mass disappears which is obtained in the form of energy (enormous amount) A + p → excited nucleus → B + C + Q.
- Nuclear Fusion: It is the phenomenon of fusing two or more lighter nuclei to form a single heavy nucleus. A + B → C + Q (Energy)
- The product (C) is more stable then reactants (A and B) and mc < (ma + mb) and mass defect ∆m = [(ma + mb) – mc] amu.
- The total binding energy and binding energy per nucleon C both are more than of A and B ∆E = Ec – (Ea + Eb).
CUET Physics Dual Nature of Radiation and Matter: Important Graphs
1. If in hydrogen atom, radius of nth Bohr orbit is rm, frequency of revolution of electron in nth orbit is fn, then
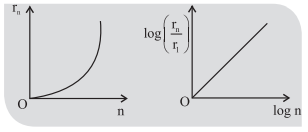
2. Law of Radioactive decay: Rate of decay of a radioactive material at any instant is proportional to quantity of that material at that time.
CUET Physics Dual Nature of Radiation and Matter: Important Formulas
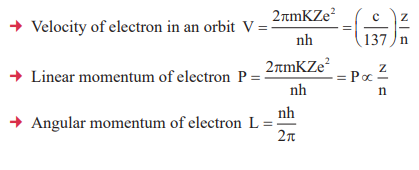
1. Outcomes of Rutherford’s Model
2. Spectral Lines
3. Atomic Properties
4. Nuclear Energy, Binding Energy, Mass Defect, Nuclear Force
5. Radioactivity, Half Life and Average Life, Activity of Radioactive Substance
6. Nuclear Reaction and Decays
CUET Physics Mock Test
Now, you have revised the this CUET Physics chapter. So, you must need to practice CUET Physics Sample Papers. By solving these CUET Physics questions, you will be more confident about your CUET preparations.