Hi CUET aspirants, Welcome to Amans Maths Blogs (AMBIPi). In this post, you will get CUET Chemistry Mock Alcohol Phenols Ethers AMBIPi. This CUET Chemistry Mock Test is of the Chemistry chapter Alcohol Phenols. Before solving these CUET Chemistry questions, you must read CUET Chemistry Notes, which helps you to revise CUET Chemistry Syllabus and then you must need to solve CUET Previous Years Questions Papers.
CUET Chemistry Mock Test
CUET Chemistry Question No: 1
Which of the following alcohol contains C sp3 − OH bond ?
Option A : Allylic alcohol
Option B : Vinylic alcohol
Option C : Phenols
Option D : None of these
Show/Hide Answer Key
Option A: Allylic alcohol
CUET Exam Chemistry Question No: 2
Vinylic alcohol contains
Option A : —OH group attached to an sp3 -hybridized carbon atom
Option B : —OH group attached to an sp3 -hybridized carbon atom
Option C : —OH group bonded to a carbon-carbon double bond
Option D : —OH group attached to sp3 -hybridised carbon atom next to an aromatic ring
Show/Hide Answer Key
Option C: —OH group bonded to a carbon-carbon double bond
Vinylic alcohols contain — OH group bonded to a carbon-carbon double bond, i.e. to a vinylic carbon or to an aryl carbon. It is as follows
H2C ≡ CH –OH
Vinylic alcohol
CUET UG Chemistry Question No: 3
The IUPAC name of following structure is
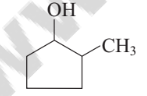
Option A : 2-methyl hydroxy cyclopentane.
Option B : 2-hydroxy 2-methyl cyclopentane
Option C : 2-methylcyclopentanol
Option D : cyclopentylmethane
Show/Hide Answer Key
Option C: 2-methylcyclopentanol
The IUPAC name of the given structure is 2-methyl cyclopentanol. Cyclic alcohols are named using the prefix cyclo and considering the —OH group attached to C-1.
CUET Domain Subject Chemistry Question No: 4
In ethers, the two bond pairs and two lone pairs of electrons on oxygen are arranged in a
Option A : planar arrangement
Option B : tetrahedral arrangement
Option C : trigonal bipyramidal arrangement
Option D : linear arrangement
Show/Hide Answer Key
Option B: tetrahedral arrangement
In ethers, the four electron pairs, i.e. the two bond pairs and two lone pairs of electrons on oxygen are arranged approximately in a tetrahedral arrangement.
CUET BSc Chemistry Question No: 5
The reagent(s) used for the reduction of aldehydes and ketone into alcohols is/are
Option A : finely divided metals such as Pt/Pd/Ni
Option B : sodium borohydride
Option C : lithium aluminium hydride
Option D : All of the above
Show/Hide Answer Key
Option D: All of the above
Fig. (a) represents structure of triclinic crystal system as, a ≠ b ≠ c and α ≠ β ≠ γ ≠ 90°. Whereas, the structures in option (b), (c) and (d) represents end-centred body centred and face-centred cubic.
CUET Entrance Exam Chemistry Question No: 6
Which of the following reagent is used to reduce carboxylic acids to primary alcohols?
Option A : Pd
Option B : R—OH
Option C : LiAlH4
Option D : Ni
Show/Hide Answer Key
Option C: LiAlH4
CUET Exam Question No: 7
Which of the following hydrocarbon is used for the world wide production of phenol?
Option A : Iso-butylbenzene
Option B : Iso-propylbenzene
Option C : Iso-pentylbenzene
Option D : None of these
Show/Hide Answer Key
Option B: Iso-propylbenzene
CUET Chemistry Practice Questions No: 8
Alcohols and phenols react with active metals to yield
Option A : alkoxides/phenoxides
Option B : hydrogen gas
Option C : nitrogen
Option D : Both (a) and (b)
Show/Hide Answer Key
Option D: Both (a) and (b)
CUET Chemistry Sample Paper Question No: 9
Arrange the following compounds in the decreasing order of acidity.
Option A : H2O>HC≡CH>ROH
Option B : H2O>ROH>HC≡CH
Option C : HC≡CH>ROH>H2O
Option D : HC≡CH>H2O>ROH
Show/Hide Answer Key
Option B: H2O>ROH>HC≡CH
CUET Chemistry Mock Test Question No: 10
Phenols show the cleavage of C— O bond with
Option A : Na
Option B : K
Option C : Zn
Option D : Ca
Show/Hide Answer Key
Option B: crystalline solids
In a cubic close packed lattice of oxide ions, there would be two tetrahedral and one octahedral void per oxide ion.
Since, the formula shows the presence of 4 oxide ions, the number of tetrahedral voids is eight and that of octahedral voids is four. Out of the eight tetrahedral voids, one is occupied by X. ∴ Percentage of tetrahedral voids occupied 1/8 × 100 =12.5 %
CUET Chemistry Question No: 11
Identify an appropriate reagent for the conversion of alcohol to carboxylic acid.
Option A : PCC
Option B : Anhydrous CrO3
Option C : Cu/573 K
Option D : KMnO4/H⊕
Show/Hide Answer Key
Option D: KMnO4/H⊕
CUET Exam Chemistry Question No: 12
Phenol on reaction with conc. HNO3 produces
Option A : o-nitrophenol
Option B : p-nitrophenol
Option C : 2, 4, 6-trinitrophenol
Option D : m-nitrophenol
Show/Hide Answer Key
Option C: 2, 4, 6-trinitrophenol
CUET UG Chemistry Question No: 13
Phenol on reaction with Zn followed by distillation gives‘ ’ X . Which on reaction with CH3COCl, AlCl3 gives Y . Final product Y is
Option A : acetophenone
Option B : benzophenone
Option C : diphenyl
Option D : methyl salicylate
Show/Hide Answer Key
Option A: acetophenone
Packing efficiency for :
Hexagonal close packing (hcp) = 74%
Face-centered cubic close packing (fcc) = 74%
Body centered cubic close packing (bcc) = 68%
and simple cubic (sc) = 52%
Thus, correct order of packing efficiency is :
hcp = fcc > bcc > sc
CUET Domain Subject Chemistry Question No: 14
Which of the following is also known as wood spirit?
Option A : Ethanol
Option B : Propanol
Option C : Methanol
Option D : Butanol
Show/Hide Answer Key
Option C: Methanol
Methanol, (CH3 OH) is known as ‘wood spirit’ as it is produced by destructive distillation of wood.
CUET BSc Chemistry Question No: 15
The action of zymase is inhibited during fermentation if the percentage of alcohol formed exceeds
Option A : 5%
Option B : 7%
Option C : 10%
Option D : 14%
Show/Hide Answer Key
Option D: 14%
CUET Entrance Exam Chemistry Question No: 16
Williamson’s synthesis involves which of the following type of mechanism when attack of an alkoxide ion on primary alkyl halide takes place?
Option A : SN1
Option B : SN2
Option C : E1
Option D : E2
Show/Hide Answer Key
Option B: SN2
Williamson’s synthesis involves S 2 N mechanism when attack of an alkoxide ion on primary alkyl halide takes place. In this mechanism, the incoming nucleophile interacts with alkyl halide causing the carbon halide bond to break while forming a new carbon —OH bond.
CUET Exam Question No: 17
Among the following sets of reactants which one produces anisole?
Option A : CH3 , CHO , RMgX
Option B : C6H5OH, NaOH, CH3I
Option C : C6H5OH , neutral FeCI3
Option D : C6H5 — CH3 , CH3COCI , AICI3
Show/Hide Answer Key
Option B: C6H5OH, NaOH, CH3I
CUET Chemistry Practice Questions No: 18
Select the correct increasing order of boiling point.
Option A : n-pentane, ethoxyethane, butan-1-ol
Option B : ethoxyethane, n-pentane, butan-1-ol
Option C : butan-1-ol, n-pentane, ethoxyethane
Option D : ethoxyethane, butan-1-ol, n-pentane
Show/Hide Answer Key
Option A: n-pentane, ethoxyethane, butan-1-ol
The weak polarity of ethers do not appreciably affect their boiling points which are comparable to those of the alkanes of comparable molecular masses but are much lower than the boiling point of alcohols. Therefore, the correct increasing order of boiling point is :
n-pentane Ethoxyethane Butan-1-ol Boiling point 309.1 < 307.6 < 390
CUET Chemistry Sample Paper Question No: 19
The order of reactivity of hydrogen halides with ether is as follows :
Option A : HBr > HI > HCl
Option B : HCl > HBr > HI
Option C : HI > HBr > HCl
Option D : HCl > HI > HBr
Show/Hide Answer Key
Option C: HI > HBr > HCl
The order of reactivity of hydrogen halides with ether is as follows. HI > HBr > HCl. The cleavage of ethers take place with concentrated HI or HBr at high temperature.
CUET Chemistry Mock Test Question No: 20
Ethers are treated with an aqueous solution of I in order to remove peroxides from it. Identify the ‘I’ from the following options.
Option A : KI
Option B : Br2
Option C : KCNS
Option D : Na2S2O3
Show/Hide Answer Key
Option A: KI
Ethers are treated with an aqueous solution of KI (I) in order to remove peroxides from it. Ether peroxide oxidises KI into I2 and itself gets reduced to ether.
