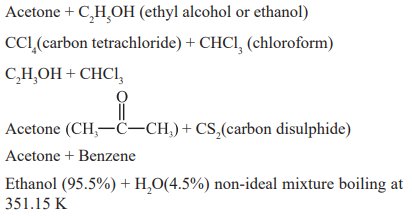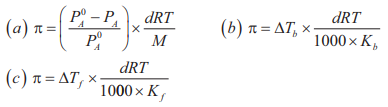Hi CUET aspirants, Welcome to Amans Maths Blogs (AMBIPi). In this post, you will get CUET Chemistry Study Materials Solutions Notes AMBIPi. This CUET Chemistry Notes are designed by analyzing to the CUET Syllabus and CUET Previous Years Questions Papers.
CUET Chemistry Notes
CUET Chemistry Solutions: Important Points to Remember
There are following important points in this chapter of Solutions.
CUET Chemistry: Introduction to Solutions
A solution is defined as a homogeneous mixture of two (or more) substances.
The solubility of all gases in water decrease with increasing temperature. That is why, carbonated drinks that has CO2
gas dissolved in them will becomes “flat” tasting when heated.
Henry’s law : States that the mass of a gas dissolved per unit volume of solvent is proportional to the pressure of the
gas in equilibrium with the solution at constant pressure . Mathematically, m ∝ p or m = kHP
Raoult’s Law : States that in a solution, the partial pressure of a component at a given temperature is equal to the mole
fraction of that component in the solution multiplied by the vapour pressure of that component in the pure state.
CUET Chemistry: Ideal & Non-ideal Solution
Ideal Solutions: The solution in which solute – solute interaction in pure state and solvent – solvent interaction in pure state are almost similar to the solute-solvent interactions.
In these solutions: ΔH (mix) = 0, ΔH (dilution) = 0, ΔH (mix) = 0, PA = PA0 ⨯ XA and PB = PB0 ⨯ XB.
Non-ideal Solutions: A non-ideal solution is that solution in which solute and solvent molecules interact with one another with a different force that the forces of interaction between the molecules of the pure components.
For such solution: ∆Hmixing ≠ 0,
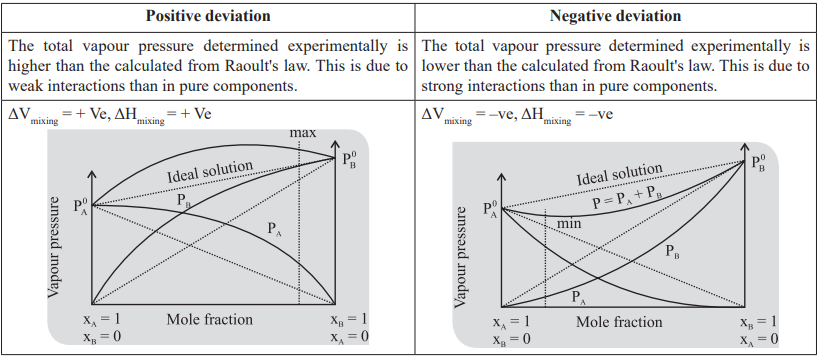
ΔV > 0. and ΔH > 0, non ideal with positive deviation
If ΔV < 0, and ΔH < 0, non ideal with negative deviation.
In terms of Raoult’s Law a non-ideal solution is that solution which does not obey Rault’s Law.
CUET Chemistry: Azeotropic Mixture
A non-ideal solution of two compounds having definite composition which boils at a constant temperature, like a pure liquid.
Minimum boiling azeotrope: For the solutions with positive deviation there is an intermediate composition for which the vapour pressure of the solution is maximum and hence, boiling point is minimum.
Maximum boiling azeotrope: For the solutions with negative deviations there is an intermediate composition for
which the vapour pressure of the solution is minimum and hence, boiling point is maximum.
Maximum boiling azeotropes.
Examples Acetone + Aniline, HCl + water, HNO3 (68%) + water (32%) non-ideal mixture boiling at 395.5 K
H2SO4 + water
Acetone + CHCl3
Minimum boiling azeotropes examples:
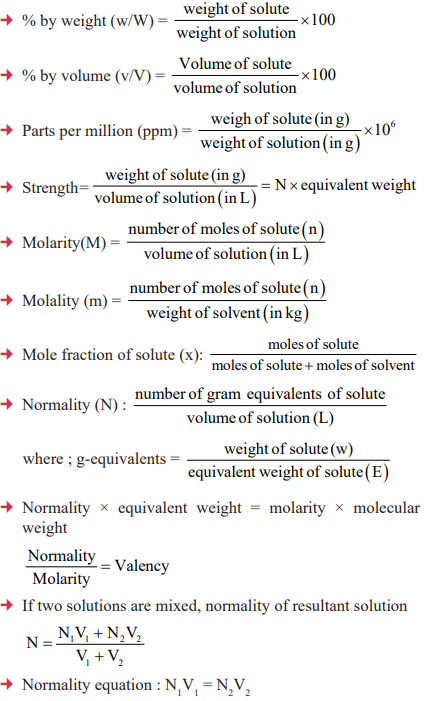
CUET Chemistry: Concentration Terms
CUET Chemistry: Colligative Properties
The properties of a solution which depend entirely upon the number of particles of the solute (ions, molecules or associated molecules) in a given volume of solvent and not on the chemical nature of the solute molecules are defined as colligative properties.
Relative lowering of Vapor Pressure: The relative lowering in vapor pressure of an ideal solution is equal to the mole fraction of solute at that temperature.
Osmotic Pressure: The excess pressure which must be applied on a solution to prevent the passage of solvent into it through a semipermeable membrane.
Elevation in Boiling Point: The temperature at which vapour pressure of the liquid becomes equal to the atmospheric pressure is known as its boiling point. The property of rise in boiling point when some non volatile solute is added.
∆Tb = Kb × m,
where Kb is molal elevation constant or ebullioscopic constant of the solvent; m = Molality of the solution, i.e., number of
moles of solute per 1000g of the solvent; ∆Tb = Elevation in boiling point.
Depression in Freezing Point or Cryoscopy: The property of decrease in freezing point when some nonvolatile solute is dissolved. The depression in freezing point is given by ∆Tf.
∆Tf = Kf × m
where Kf = molal depression constant or cryoscopic constant; m = Molality of the solution (i.e., no. of moles of solute per
of the solvent); ∆Tf = Depression in freezing point
Freezing Point: Temperature at which the liquid and the solid forms of the same substance are in equilibrium and hence have same vapour pressure.
Relation of osmotic pressure with different colligative properties: Osmotic pressure is related to relative lowering of vapour pressure, elevation of boiling point and depression of freezing point according to the following relations:
In the above relations, π = Osmotic pressure; d = Density of solution at temperature T; R = Universal gas constant; M = Mol. Mass of solute; Kb = Molal elevation constant of solvent; Kf = Molal depression constant of solvent
CUET Chemistry: Van’t Hoff’s factor (i)
CUET Chemistry: Reverse Osmosis (R.O)
When pressure applied on solution is more than osmotic pressure, solvent will start flowing from solution to pure solvent, through the semi-permeable membrane. The process is called Reverse Osmosis.
An important application of reverse osmosis is in the desalination of sea water i.e. removal of salts from sea water.
CUET Chemistry: Some Other Important Points
Some substances can loose water of crystallization partially or completely on exposure to air. The phenomenon is called efflorescence and the substance called efflorescent substance
Na2SO4.7H2O → Na2SO4.3H2O
Deliquescence Some substance can absorb moisture from air and get dissolved in it. As the vapour pressure of the saturated solution of that substance is less then vapour pressure of air.
MgCl2 + 6H2O → MgCl2.6H2O
Pure NaCl neither effloresce nor deliquesces under normal condition. But in presence of impurities like MgCl2 or CaCl2
it damps due to absorption of water.
With increase in temperature the vapour pressure of the substance increases & efflorescence is favoured. Copper sulphate neither effloresce nor deliquescent.
When a non-volatile solute is added to a solvent the vapour pressure is lowered, due to the following reasons, this proccess is known as lowering in vapour pressure.
Percentage surface occupied by a solvent decreases. Thus; the rate of evaporation and vapour pressure decreases.
According to Graham’s law of evaporation,
Two solution of different substances having same osmotic pressure at same temperature are known as isotonic solutions.
A solution having osmotic pressure than the other solution are known as hypertonic solution.
CUET Chemistry Mock Test
Now, you have revised the this CUET Chemistry chapter. So, you must need to practice CUET Chemistry Sample Papers. By solving these CUET Chemistry questions, you will be more confident about your CUET preparations.