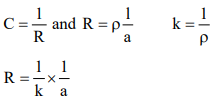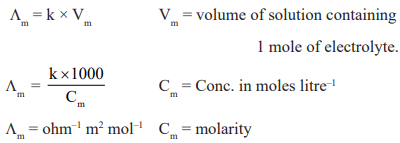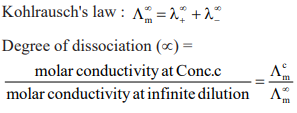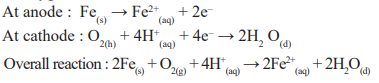Hi CUET aspirants, Welcome to Amans Maths Blogs (AMBIPi). In this post, you will get CUET Chemistry Study Materials Electrochemistry Notes AMBIPi. This CUET Chemistry Notes are designed by analyzing to the CUET Syllabus and CUET Previous Years Questions Papers.
CUET Chemistry Notes
CUET Chemistry Electrochemistry: Important Points to Remember
There are following important points in this chapter of Electrochemistry.
CUET Chemistry: Introduction to Electrochemistry
The study of relationship between electrical energy and chemical energy, produced in a redox reaction, and how one can be converted into another is known as electrochemistry.
Electrochemical cell: Electrochemical cell is system or arrangement in which two electrodes are fitted in the same electrolyte or in two different electrolytes which are joined by a salt bridge.
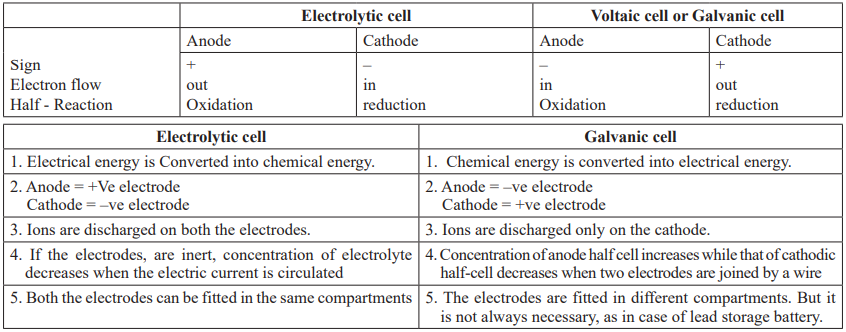
Electrolytic Cell: In this cell electrical energy is converted into chemical energy & electrolysis is carried out by passing electricity. Electrolytic cell contains two electrodes that dip into an electrolyte & are connected to a battery (galvanic cell) or some other source of direct current. The process of decomposition of an electrolyte by passing electricity is called electrolysis.
Galvanic Cell: Galvanic cell is an electrochemical cell that converts the chemical energy of a spontaneous redox reaction into electrical energy. In this device the Gibbs energy of the spontaneous redox reaction is converted into electrical work.
Electrode potential: When a metal is placed in a solution of its ions the metal acquires either a positive or negative charge with respect to the solution. On account of this a definite potential difference is developed between metal and the solution. This potential difference is called electrode potential.
CUET Chemistry: Electrochemical Cell and Gibbs Free Energy
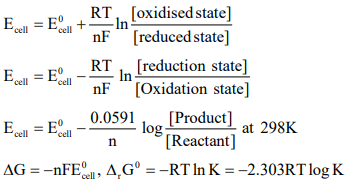
To determine standard free energy change (ΔG0) of the reaction.
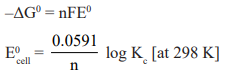
If the standard EMF (E0) is known, then as the equilibrium constant of the cell reaction can be calculated by the use of
equation. –ΔG0 = RTlnK = nFE0.
CUET Chemistry: Nernst Equation
The relationship of electrolyte concentration of electrode potential of the cell is shown by this equation.
Ohm’s Law: Ohm’s Law relates the current in ampere (i) to potential difference (E) applied across the conductor and the
resistance (R) of the conductor i = E/R.
Conductance (C): C = 1 / R
Specific conductance (K): Specific conductance of a conductor is the conductance of ions shown unit volume of an electrolyte.
1/A = = Cell constant, depends upon the dimensions of the cell
Equivalent conductance (Λeq): The conducting power of all the ions produced by one gram equivalent of an electrolyte in the solution is defined by equivalent conductance.
Molar Conductance (Λm): It is defined as the conducting power of all the ions produced by one mole of the electrolyte
in solution.
CUET Chemistry: Faraday’s Laws & Commercial Cells
Faraday’s 1st Law of Electrolysis: The mass of substance deposited is directly proportional to charge passed through electrolyte. m ∝ Q, where Q is charge. m = z × I × t, where I is current in ampere, ‘t’ is time in second, ‘z’ is electro chemical equivalent.
Faraday’s second law of electrolysis: It states when equal charge is passed through different electrolytes, amount
of substance deposited is proportional to their equivalent weights.
Commercial Cells: Primary Cells are those in which the redox reaction occurs only once and the cell becomes dead after sometime e.g., dry cell, mercury cell.
A). Secondary Cells are those which can be recharged by passing an electric current through them and hence can be
used over and again e.g., lead storage.
B). Fuel Cells are those in which the energy produced from the combustion of fuels like H2, CO, CH4 etc. is converted
into electric current.
CUET Chemistry: Electrolytic Cell & Galvanic Cell
CUET Chemistry: Batteries & Corrosion
Batteries: The arrangement of no. of cells connected in series is called battery. Examples of some commercial cells batteries) are given. Primary cells (eg: Dry cell, mercury cell) secondary cell (eg : lead storage battery, nickel-cadmium cell) fuel cell(eg: lead storage batter, nickel- cadmium cell) fuel cell (eg: H2 – O2 fuel cell).
Efficiency of fuel cell is.
Corrosion: Corrosion is the slow eating away of metals when exposed to the atmosphere.
Corrosion of Iron (Rusting) : Is is an electrochemical phenomenon and oxygen occurs in the presence of moisture and oxygen.
Methods used for prevention of corrosion are barrier protection, sacrificial protection, anti-rust solutions.
CUET Chemistry Mock Test
Now, you have revised the this CUET Chemistry chapter. So, you must need to practice CUET Chemistry Sample Papers. By solving these CUET Chemistry questions, you will be more confident about your CUET preparations.