Hi CUET aspirants, Welcome to Amans Maths Blogs (AMBIPi). In this post, you will get CUET Chemistry Mock Test General Principle Process of Isolation of Elements AMBIPi. This CUET Chemistry Mock Test is of the Chemistry chapter General Principle Process of Isolation of Elements. Before solving these CUET Chemistry questions, you must read CUET Chemistry Notes, which helps you to revise CUET Chemistry Syllabus and then you must need to solve CUET Previous Years Questions Papers.
CUET Chemistry Mock Test
CUET Chemistry Question No: 1
What percentage of carbon is found in wootz steel?
Option A : 2-2.5%
Option B : 1.0-1.9%
Option C : 3-4.5%
Option D : 0.5-1%
Show/Hide Answer Key
Option D: 1.0-1.9%
Wootz steel contains high proportion of carbon that ranges between 1.0% and 1.9 %
CUET Exam Chemistry Question No: 2
Which of the following elements occurs in free state?
Option A : Iodine
Option B : Sulphur
Option C : Phosphorus
Option D : Magnesium
Show/Hide Answer Key
Option C: Sulphur
A few elements like carbon, sulphur, gold and noble gases occur in free state. Iodine, phosphorus and magnesium are reactive and found in combined state.
CUET UG Chemistry Question No: 3
The ore that contains both iron and copper is
Option A : Malachite
Option B : Azurite
Option C : Dolomite
Option D : Copper Pyrites
Show/Hide Answer Key
Option D: Copper pyrites
Zincite (ZnO) And cuprite (Cu2O) are the examples of oxide ores while copper glance (Cu2S) is an example of sulphide ores.
CUET Domain Subject Chemistry Question No: 4
Which one of the following is a mineral of iron?
Option A : Malachite
Option B : Cassiterite
Option C : Pyrolusite
Option D : Magnetite
Show/Hide Answer Key
Option D: Magnetite
(A)KNO3 – Orthorhombic; (B)CaCO3 – Trigonal; (C) CaSO4 – Tetragonal; (D) CuSO4.5H2O – Triclinic
CUET BSc Chemistry Question No: 5
The froth stabilizers among the following are
Option A : Pine oil
Option B : Cresol
Option C : Aniline
Option D : Both (b) and (c)
Show/Hide Answer Key
Option D: Both (b) and (c)
CUET Entrance Exam Chemistry Question No: 6
An ore contains lead sulphide and zinc sulphide. If froth floatation process is used, these can be separated
Option A : By using excess of pine oil
Option B : By using collection and froth stabilizers
Option C : By adjusting proportion of oil to water or using depressants
Option D : By using some suitable solvent in which either lead sulphide or zinc sulphide is soluble.
Show/Hide Answer Key
Option C: by adjusting proportion of oil to water or using
depressants
The given ore contains lead sulphide and zinc sulphide. It is possible to separate two sulphide ores by adjusting proportion of oil to water or by using depressants. The depressant used in the case of given ore is NaCN.
CUET Exam Question No: 7
Froth floatation process for the concentration of ores is an illustration of the practical application of
Option A : Absorption
Option B : Adsorption
Option C : Sedimentation
Option D : Coagulation
Show/Hide Answer Key
Option B: adsorption
CUET Chemistry Practice Questions No: 8
The ore which is concentrated by leaching process is
Option A : Cuprite
Option B : Argentite
Option C : Sphalerite
Option D : Haematite
Show/Hide Answer Key
Option B: argentite
Argentite and bauxite are the ores of less reactive and highly reactive metals and are soluble in some suitable reagent, that’s why these are concentrated by leaching. Thus, among the given ores, the one which is concentrated by leaching process.
CUET Chemistry Sample Paper Question No: 9
Extraction of gold and silver involves leaching with CN−ion. Silver is later recovered by
Option A : Liquation
Option B : Distillation
Option C : Zone refining
Option D : Displacement with Zn
Show/Hide Answer Key
Option D: displacement with Zn
Extraction of gold and silver involves leaching with CN−ion. Silver is later recovered by displacement of zinc (Zn). In the metallurgy of silver or gold, the respective metal is leached with a dilute solution of NaCN or KCN in the presence of air to obtain the metal complex in solution. From the complex, metal is obtained through the displacement reaction. In general,
4M (s) + 8 CN– (aq) + 2H2O (aq) + O2 (g) → 4 [M (CN)2] (aq) + 4 OH- (aq) 2[M (CN2)]- (aq)+ Zn(s)→ [Zn(CN)4]2– (aq) + 2M (s)
M= Ag or Au
This method is known as MacArthur-Forrest cyanide process.
CUET Chemistry Mock Test Question No: 10
Which of the following is not true in the context of roasting process?
Option A : The ore is heated in a regular supply of air
Option B : The sulphide ores of copper are heated in reverberatory furnace
Option C : 2ZnS + 3O2→ 2ZnO + 2 SO2 is a reaction that involves roasting process
Option D : Mg, Al and Zn oxides can be reduced by roasting process
Show/Hide Answer Key
Option D: Mg, Al and Zn oxides can be reduced by roasting process
Due to comparatively higher reactivity, oxides of Mg, Al and Zn can not be reduced by roasting process. In case of such oxides electrolytic process is used.
CUET Chemistry Question No: 11
If the sulphide ores of copper contains iron, then before heating, it is mixed with
Option A : Silica
Option B : Coke
Option C : Carbon
Option D : Coal
Show/Hide Answer Key
Option A: silica
If the sulphide ores of copper ore contains iron, it is mixed with silica before heating. Iron oxide ‘slags off’ as iron silicate and copper is produced in the form of copper matte which contains Cu2S and FeS.
CUET Exam Chemistry Question No: 12
Sulphide ores are common for which of the following metals.
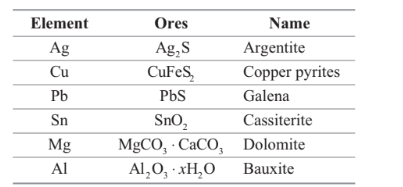
Option A : Ag, Cu and Pb
Option B : Ag, Cu and Sn
Option C : Ag, Mg and Pb
Option D : Al, Cu and Pb
Show/Hide Answer Key
Option A: Ag, Cu and Pb
Thus, sulphide ores are common for the metals Ag, Cu and Pb.
CUET UG Chemistry Question No: 13
Roasting of sulphides gives the gas X as a by-product. This is a colourless gas with choking smell of burnt sulphur and causes great damage to respiratory organs as a result of acid rain. Its aqueous solution is acidic acts as a reducing agent and its acid has never been isolated. The gas X is
Option A : H2S
Option B : SO2
Option C : CO2
Option D : SO3
Show/Hide Answer Key
Option D: SO3
Less electropositive metals like copper, mercury and lead involve auto-reduction in their extraction process. Auto-reduction is not involved in the extraction of aluminium as it is more electropositive.
CUET Domain Subject Chemistry Question No: 14
Auto-reduction is not involved in the extraction of
Option A : Copper
Option B : Mercury
Option C : Lead
Option D : Aluminium
Show/Hide Answer Key
Option A: Copper
CUET BSc Chemistry Question No: 15
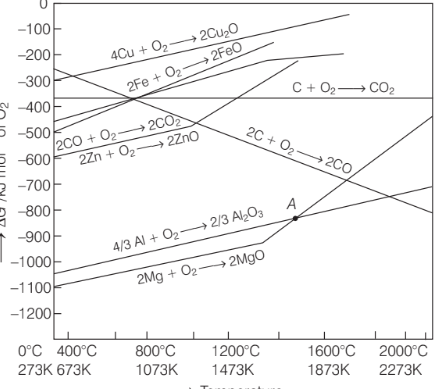
Considering Ellingham diagram, which of the following metals can be used to reduce alumina?
Option A : Mg
Option B : Zn
Option C : Fe
Option D : Cu
Show/Hide Answer Key
Option A: Mg
From the Ellingham diagram the plot for the formation of MgO intersects the plot for the formation of Al2 O3 at around 1500°C.
Thus, Mg can be use to reduce alumina at that temperature.
CUET Entrance Exam Chemistry Question No: 16
The reducing agent used to reduce iron oxide in blast furnace is
Option A : silica
Option B : CO
Option C : C
Option D : lime
Show/Hide Answer Key
Option B: CO
Carbon monoxide is the reducing agent which reduces iron oxide (Fe2O23) to iron in the blast furnace.
Fe203 + 3 CO → 2 Fe + 3O2
CUET Exam Question No: 17
Which of the following elements is present as the impurity to the maximum extent in the pig iron?
Option A : Carbon
Option B : Silicon
Option C : Phosphorus
Option D : Manganese
Show/Hide Answer Key
Option A: Carbon
Pig iron contains about 4% carbon (major impurity) and other impurities (S, P, Si, Mn) in trace amounts.
CUET Chemistry Practice Questions No: 18
Silica is added to the copper pyrites ore, when taken in reverberatory furnace for extraction of Cu. This is because
Option A : it removes the impurity of iron oxide as slag
Option B : it reacts with Cu2O to form slag
Option C : it reduces Cu2O to Cu
Option D : it helps in separation of Cu from Fe
Show/Hide Answer Key
Option A: it removes the impurity of iron oxide as slag
Silica is added to the copper pyrites ore when taken in reverberatory furnace for extraction of copper because silica removes the impurity of FeO present in the copper pyrites as slag (FeSiO3).
CUET Chemistry Sample Paper Question No: 19
In the extraction of copper from its sulphide ore, the metal finally obtained by the reduction of cuprous oxide with
Option A : copper (I) sulphide (Cu2S )
Option B : sulphur dioxide ( SO2)
Option C : iron sulphide (FeS)
Option D : carbon monoxide (CO)
Show/Hide Answer Key
Option A: copper (I) sulphide (Cu2S)
CUET Chemistry Mock Test Question No: 20
In electrolytic refining method,
Option A : the impure metal is made to act as anode
Option B : a strip of the pure metal is used as cathode
Option C : anode and cathode are kept in a suitable electrolytic bath containing soluble salt of the same metal
Option D : All the above are true
Show/Hide Answer Key
Option D: All the above are true
In electrolytic refining method, the impure metal is made to act as anode. A strip of the same metal in pure form is used as cathode. They are kept in a suitable electrolytic bath containing soluble0 salt of the same metal. The more basic metal remains in the solution and the less basic ones go to the anode mud.
Anode M → Mn+ + ne–
Cathode Mn+ + ne–→ M