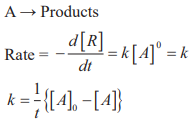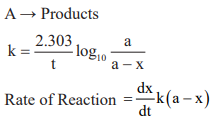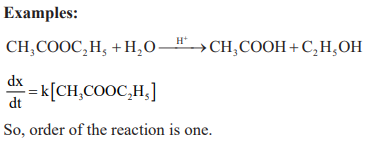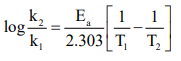Hi CUET aspirants, Welcome to Amans Maths Blogs (AMBIPi). In this post, you will get CUET Chemistry Notes Study Materials Chemical Kinetics AMBIPi. This CUET Chemistry Notes are designed by analyzing to the CUET Syllabus and CUET Previous Years Questions Papers.
CUET Chemistry Notes
CUET Chemistry Chemical Kinetics: Important Points to Remember
There are following important points in this chapter of Chemical Kinetics.
CUET Chemistry: Introduction to Chemical Kinetics
The branch of chemistry which deals with rate and mechanism of chemical reaction is known as chemical kinetics.
Rate of reaction is be expressed as the change in concentration of any one reactant or product per unit time. A → B

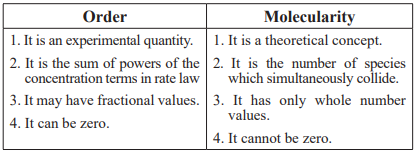
CUET Chemistry: Order & Molecularity
Collision frequency (Z) is the number of collision that takes place per second per unit volume of a reaction mixture Z ∝ √T.
The collisions which actually produce the products and result in the chemical reaction are effective collision.
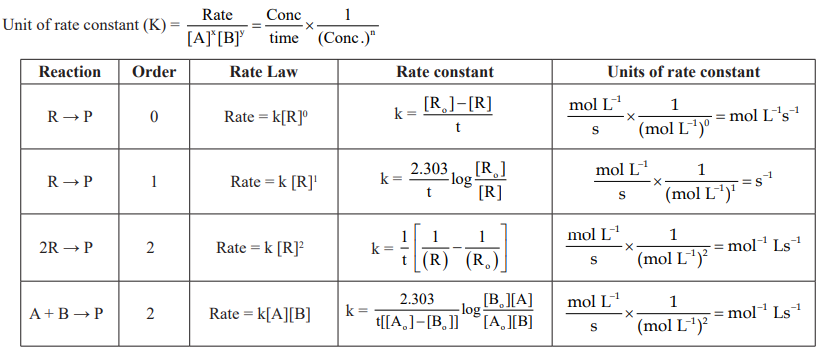
CUET Chemistry: Rate of a Chemical Reaction
CUET Chemistry: Factor Influence Rate of a Chemical Reaction
Concentration: Faster is the rate of reaction when greater the concentrations of the reactants.
Physical State of Reactants: Reactions involving gaseous reactants are faster than reactions containing liquid reactants.
Temperature: The increase of temperature result in the increase of rate of reaction for most of the reactions, rate of reactions becomes almost double with 10°C rise of temperature.
Presence of Catalyst: A catalyst generally increases the speed of a reaction.
Surface area of Reactants: For a reaction involving a solid reactant or catalyst, the greater is the surface , the faster is
the rate of reaction.
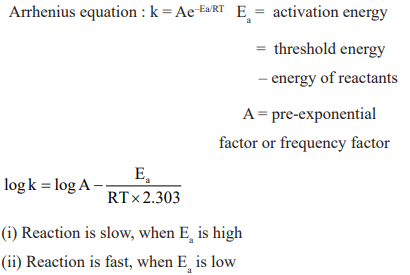
Activation Energy: Lower the activation energy, faster is the reaction.
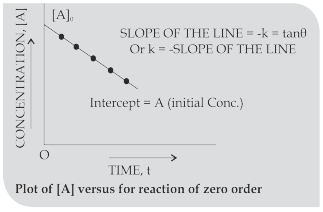
CUET Chemistry: Zero Order, Half Life Period, Pseudo-Unimolecular Reactions
Zero order reactions:
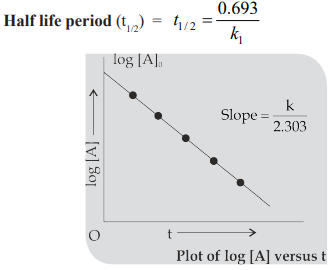
Half life period (t1/2): The time in which half of the initial amount of reactants is converted to products or the time taken for 50% completion of reaction is known as half life period. For zero order reaction A → Products
First order reaction:
Pseudo – Unimolecular reactions: Those first order reactions whose molecularity is not one are called pseudo-nimolecular reactions.
CUET Chemistry: Temperature Dependence of the Rate of Reaction
CUET Chemistry: Collision Theory of Chemical Reaction
CUET Chemistry Mock Test
Now, you have revised the this CUET Chemistry chapter. So, you must need to practice CUET Chemistry Sample Papers. By solving these CUET Chemistry questions, you will be more confident about your CUET preparations.