Hi CUET aspirants, Welcome to Amans Maths Blogs (AMBIPi). In this post, you will get CUET Chemistry Notes Study Materials p Block Elements AMBIPi. This CUET Chemistry Notes are designed by analyzing to the CUET Syllabus and CUET Previous Years Questions Papers.
CUET Chemistry Notes
CUET Chemistry The p-Block Elements: Important Points to Remember
There are following important points in this chapter of p-Block Elements.
CUET Chemistry: Group 15 Elements
Electronic configuration: ns2 np3
Oxidation State: They exhibit two important oxidation states, + 3 and + 5 but +3 oxidation is favored by heavier elements due to ‘inert pair effect’.
Ionization Enthalpy: Decreases from Nitrogen (N) to Bismuth (Bi) due to increase in atomic size.
Metallic character: It increases down the group as the ionization energy decreases.
Nitrogen: Nitrogen differs from rest of the elements of this group due to its small size, high electronegativity, high ionization energy, and non-availability of d-orbitals and formation of p–p multiple bonds with itself and with highly electronegative atom like O or C.
Dinitrogen: Dinitrogen is a colourless, odourless, tasteless and non-toxic gas. The main use of dinitrogen is in the manufacture of ammonia and other industrial chemicals containing nitrogen, (e.g., calcium cyanamide).
Ammonia: Ammonia is a colourless gas with a pungent odour. Its freezing and boiling points are 198.4 and 239.7 K respectively. Ammonia is used to produce various nitrogenous fertilisers (ammonium nitrate, urea, ammonium phosphate and ammonium sulphate) and in the manufacture of some inorganic nitrogen compounds, the most important one being nitric acid. Liquid ammonia is also used as a refrigerant.
Nitric Acid: The major use of nitric acid is in the manufacture of ammonium nitrate for fertilisers and other nitrates for use in explosives and pyrotechnics.
Halides: It forms two types of halides as PX3 and PX5. PCl3 is prepared by the reaction of white phosphorus with dry chlorine. P4 + 6Cl2 🡒 4PCl3.
While PCl5 is prepared by the reaction of phosphorus with SO2Cl2. P4 + 10SO2Cl2 🡒 4PCl5 + 10SO2.
Phosphorus: Phosphorus forms a number of oxoacids. Depending upon the number of P–OH groups, their basicity varies. The oxoacids which have P–H bonds are good reducing agents.
CUET Chemistry: Group 16 Elements
Electronic configuration: ns2 np4
Atomic and Ionic radii: Due to increase in the number of shells, atomic and ionic radii increase from top to bottom in the group. The size of oxygen atom is, however, exceptionally small.
Ionization Enthalpy: Decreases down the group. It is due to increase in size.
Electronegativity: Decreases down the group.
Oxidation state: They show +2, +4, and +6 oxidation states. Only oxygen shows an oxidation state of -2 (except of OF2 and H2O2).
Dioxygen: Dioxygen is a colourless and odourless gas, it directly reacts with nearly all metals and nonmetals except some metals ( e.g., Au, Pt) and some noble gases.
Its combination with other elements is often strongly exothermic which helps in sustaining the reaction.
In laboratory, dioxygen is prepared by heating KClO3 in presence of MnO2.
It forms a number of oxides with metals and they are classified on the basis of chemical nature. For example, Metallic oxides (Na2O, CaO etc), Nonmetallic (CO2, SO2 etc), amphoteric oxides (SnO2, Al2O3 etc).
Allotropic form of oxygen: Allotropic form of oxygen is O3, which is a highly oxidizing agent.
Sulphur: Sulphur forms a number of allotropes. Of these, – and – forms of sulphur are the most important. Sulphur combines with oxygen to give oxides such as SO2 and SO3. SO2 is prepared by the direct union of sulphur with oxygen.
SO2 is used in the manufacture of H2SO4.
CUET Chemistry: Group 17 Elements
Electronic configuration: ns2 np5
Atomic and Ionic radii: They have the smallest radii in their respective periods because of increase in nuclear charge. It increases down the group.
Ionization Enthalpy: Decreases down the group.
Electronegativity: Decreases down the group and they are the most electronegative elements in their respective periods.
Oxidation state: Except fluorine, other elements show oxidation states of +1, +3, +5 and +7. Fluorine shows -1 oxidation state.
Melting and boiling point: It increases as we move down the group due to increase in radii and nuclear charge which causes greater van der Waal’s forces of attraction.
These elements are extremely reactive and as such they are found in the combined state only. They form oxides, hydrogen halides, interhalogen compounds and oxoacids.
Acidic strength of Hydrogen halides: HF < HCl < HBr < HI.
Most of the oxides formed by these halogens are unstable and their stability decreases in the order I > Cl > Br.
Acidic strength of oxoacids containing different halogen: HClO > HBrO > HIO
Acidic strength of oxoacids containing the same halogen: HClO < HClO2 < HClO3 < HClO4
Properties of Chlorine: Greenish yellow gas with a pungent suffocating smell, soluble in water
CUET Chemistry: Group 18 Elements
Electronic configuration: ns2 np6
Atomic and Ionic radii: They have the largest radii in their respective periods and it increases down the group.
Ionization Enthalpy: Decreases down the group and have highest ionization enthalpy in their respective periods.
Melting and boiling point: Low melting and boiling points because of weak van der Waal’s forces. Increases down the group.
Complete Octet: Due to complete octet of outermost shell, they have less tendency to form compounds. The best characterised compounds are those of xenon with fluorine and oxygen only under certain conditions.
These gases have several uses. Argon is used to provide inert atmosphere, helium is used in filling balloons for meteorological observations, neon is used in discharge tubes and fluorescent bulbs.
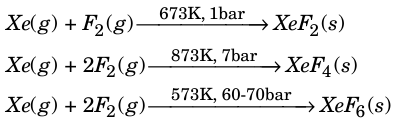
Xenon forms three binary fluorides, XeF2, XeF4 and XeF6 by following reactions.
Xenon trioxide (XeO3)
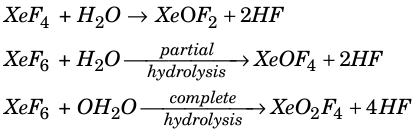
Xenon oxyfluorides
CUET Chemistry Mock Test
Now, you have revised the this CUET Chemistry chapter. So, you must need to practice CUET Chemistry Sample Papers. By solving these CUET Chemistry questions, you will be more confident about your CUET preparations.