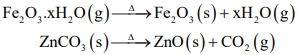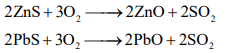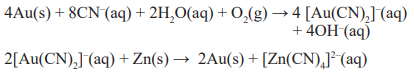Hi CUET aspirants, Welcome to Amans Maths Blogs (AMBIPi). In this post, you will get CUET Chemistry Notes General Principles and Processes of Isolation of Elements AMBIPi. This CUET Chemistry Notes are designed by analyzing to the CUET Syllabus and CUET Previous Years Questions Papers.
CUET Chemistry Notes
CUET Chemistry General Principles and Processes of Isolation of Elements: Important Points to Remember
There are following important points in this chapter of General Principles and Processes of Isolation of Elements.
CUET Chemistry: Introduction to Minerals, Ores, Gangue, Metallurgy
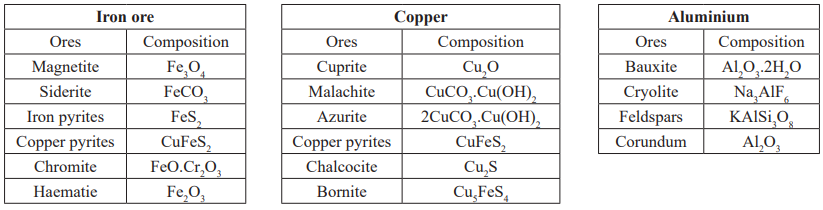
Minerals: Naturally occurring chemical substances in earth’s crust obtained by mining.
Ores: Minerals in which a metal may be found in greater concentration.
Gangue: Rarely an ore contains only a desired substance. It is usually contaminated with earthly or undesired materials
known as gangue.
Metallurgy: The entire scientific and technological process used for isolation of the metal from its ores is known as metallurgy.
All minerals are not ores but all ores are minerals.
CUET Chemistry: Concentration of Ores or Ore Dressing
Concentration is done by:
Hydraulic washing – type of gravity separation
Froth – flotation process – used for sulphides
Magnetic separation – for magnetic ores.
Electrostatic separation – eg: to separate lead sulphide and zinc sulphide
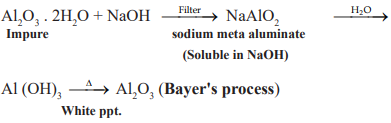
Chemical method – eg: Baeyer’s process used for BAUXITE.
CUET Chemistry: Leaching of Alumina from Bauxite
Alumina is leached by Hall’s – Heroult process, Bayer’s process, Serpeck’s process
Temp 473-523 K
Pressure 35-36 bar
Al2O3 is leached out as sodium aluminate (and SiO2 too as sodium silicate) leaving the impurities behind.
CUET Chemistry: Calcination
Process of heating of concentrated ore in absence of air or in limited supply of air below its melting point.
Roasting: It is the process of heating of concentrated ore in presence of excess of air below its melting point with or without the addition of external substance. During roasting, moisture get removed, impurities of sulphur, arsenic, antimony removed as their volatile oxides and also the sulphide ore is converted into oxide ore.
CUET Chemistry: Goldschmidt’s Thermic Process
During this process, thermite is used, which is a mixture of metal oxide of less electropositive metal like cobalt, Mo, Mn,
Fe, etc. and aluminium powder. This process is generally used for reduction of oxide of less electropositive metal because these are not easily reduced by carbon due to high melting point.
CUET Chemistry: Ellingham Diagram
The graphical representation of Gibbs energy.
Each plot is a straight line except when some change in phase (s → liq or liq → g) takes place. The temperature, at which such change occurs, is indicated by an increase in the slope on +ve side (e.g., in the Zn, ZnO plot, the melting is indicated by an abrupt change in the curve).
Limitations of Ellingham Diagram: The graph simply indicates whether a reaction is possible or not. It does not say about the kinetics of the reduction process.
The interpretation of is based on ΔK ( = – RT lnK). Thus it is presumed that the reactants and products are in equilibrium:
CUET Chemistry: Copper Extraction
Copper is extracted by hydrometallurgy from low grade ores. It is leached out using acid or bacteria. The solution containing Cu2+ is treated with scrap iron or H2.
Gold and Silver Extraction
CUET Chemistry: Zone Refining & Vapour Phase Refining
Zone Refining: This method is based on the principle that the impurities are more soluble in the melt than in the solid state of the metal.
This method is very useful for producing semiconductor and other metals of very high purity, e.g., germanium, silicon, boron, gallium and indium.
Vapour Phase Refining: Mond Process for Refining Nickel: In this process, nickel is heated in a stream of carbon monoxide forming a volatile complex, nickel tetracarbonyl:
The carbonyl is subjected to higher temperature so that it is decomposed giving the pure metal; this indicates that complex is highly instable at higher temperatures.
Van Arkel Method for Refining Zirconium or Titanium: This method is very useful for removing all the oxygen and nitrogen present in the form of impurity in certain metals like Zr and Ti. The crude metal is heated in an evacuated vessel with iodine. The metal iodide being more covalent, volatilises:
The metal iodide is decomposed on a tungsten filament, electrically heated to about 1800 K. The pure metal is thus deposited on the filament.
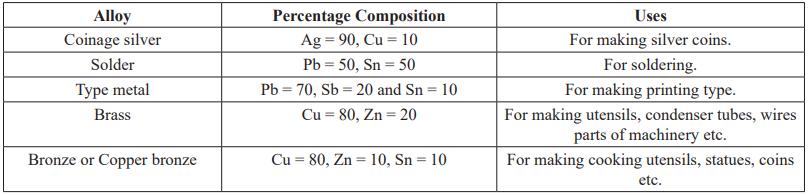
CUET Chemistry: Alloys
A metallic product containing two or more metals or sometimes one of the ingredients a non metal provided that the mixture is homogeneous and possesses metallic properties.
Casting : An alloy of lead and antimony is known as type metal is used for casting type required in printing works.
CUET Chemistry Mock Test
Now, you have revised the this CUET Chemistry chapter. So, you must need to practice CUET Chemistry Sample Papers. By solving these CUET Chemistry questions, you will be more confident about your CUET preparations.